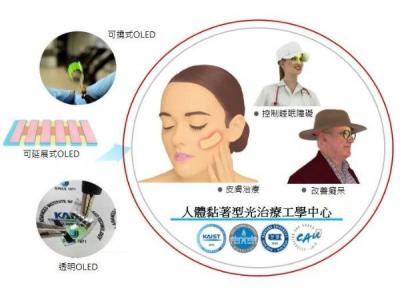
A few weeks ago Korea-based Sewon announced that it is entering the OLED-based optical patch business, based on technology developed by Kwangbio and Korea's KAIST institute. The idea is to use OLED panels for phototherapy that can be used to treat pain and encourage skin regeneration.
Sewon E&C now announced that its OLED phototherapy patch, the first such patch in the world, has completed the FDA registration. The company now aims to accelerate the commercialization, and has already secured the site for the production facility. Sewon hopes to bring the patch to the market by the end of 2022 or early in 2023.
The OLED optical patch developed a KAIST is a free-form platform that can be attached to the body. It is based on flexible OLED panels and is less than 1 mm thick. The patches have been tested with preclinical trials in the first half of 2022, which achieved excellent results. Sewon plans to expand the application of the patches to more fields, such as dementia, skin cancer, skin regeneration, wrinkle improvement, and other skin diseases.
Sewon E&€™s OLED phototherapy patch is less than 1 mm thick, weighs less than 1 gram and can be flexed with a radius of 20 mm. It has a lifetime of over 300 hours.
Posted: Sep 12,2022 by Ron Mertens

